Transparent window vibrational probes for the characterization of proteins with high structural and temporal resolution
R. Adhikary, J. Zimmermann, F.E. Romesberg, Chem. Rev. (2017) 117:1927–1969.
 We review the strengths and weaknesses of the different transparent window vibrational probes, methods by which they may be site-specifically incorporated into peptides and proteins, and the physicochemical properties they may be used to study. These topics are put into context through four case studies focused on KSI, SH3, DHFR, and cyt c.
We review the strengths and weaknesses of the different transparent window vibrational probes, methods by which they may be site-specifically incorporated into peptides and proteins, and the physicochemical properties they may be used to study. These topics are put into context through four case studies focused on KSI, SH3, DHFR, and cyt c.
 In this work, three affinity matured anti-MPTS antibodies (Abs) are characterized via X-ray and spectroscopic studies and the results are discussed in terms of the elasticity, anelasticity, and plasticity of the complexes. We find that the level of plasticity of the Ab-MPTS complexes is correlated with specificity, suggesting that the optimization of protein dynamics may have contributed to affinity maturation.
In this work, three affinity matured anti-MPTS antibodies (Abs) are characterized via X-ray and spectroscopic studies and the results are discussed in terms of the elasticity, anelasticity, and plasticity of the complexes. We find that the level of plasticity of the Ab-MPTS complexes is correlated with specificity, suggesting that the optimization of protein dynamics may have contributed to affinity maturation. We characterize the IR stretching frequencies of deuterated variants of proline and four proline residues of an Src homology 3 domain protein. CD stretching frequencies are shifted to lower energies due to hyperconjugation with Ni electron density, and along with DFT calculations, the data reveal that the Ni+1-H–Ni interactions constitute H-bonds that may play an important role in protein folding, structure, and function.
We characterize the IR stretching frequencies of deuterated variants of proline and four proline residues of an Src homology 3 domain protein. CD stretching frequencies are shifted to lower energies due to hyperconjugation with Ni electron density, and along with DFT calculations, the data reveal that the Ni+1-H–Ni interactions constitute H-bonds that may play an important role in protein folding, structure, and function. We use IR spectroscopy to characterize nSH3 variants labeled with CN or N3 at five different positions. Like carbon-deuterium (C-D) bonds, CN and N3 can provide information about their surrounding protein environment, but unlike C-D, they tend to be perturbative and thus should be used with caution.
We use IR spectroscopy to characterize nSH3 variants labeled with CN or N3 at five different positions. Like carbon-deuterium (C-D) bonds, CN and N3 can provide information about their surrounding protein environment, but unlike C-D, they tend to be perturbative and thus should be used with caution. We describe the experimental procedures required to use C-D bonds and FT IR spectroscopy to characterize protein dynamics, structural and electrostatic heterogeneity, ligand binding, and folding.
We describe the experimental procedures required to use C-D bonds and FT IR spectroscopy to characterize protein dynamics, structural and electrostatic heterogeneity, ligand binding, and folding. We use the IR absorptions of carbon-deuterium bonds site-selectively incorporated throughout the N-terminal SH3 domain from the murine adapter protein CrkII to characterize its different microenvironments with high spatial and temporal resolution.
We use the IR absorptions of carbon-deuterium bonds site-selectively incorporated throughout the N-terminal SH3 domain from the murine adapter protein CrkII to characterize its different microenvironments with high spatial and temporal resolution.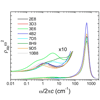 We report the sequence, thermodynamic, and time-resolved spectroscopic characterization of a panel of eight antibodies (Abs) elicited to a chromophoric antigen (MPTS). Three of the Abs arose from unique germline Abs, while the remaining five comprise two sets of siblings that arose by somatic mutation of a common precursor. By characterizing both flexibility and conformational heterogeneity, we show that point mutations are capable of fixing significant differences in protein dynamics.
We report the sequence, thermodynamic, and time-resolved spectroscopic characterization of a panel of eight antibodies (Abs) elicited to a chromophoric antigen (MPTS). Three of the Abs arose from unique germline Abs, while the remaining five comprise two sets of siblings that arose by somatic mutation of a common precursor. By characterizing both flexibility and conformational heterogeneity, we show that point mutations are capable of fixing significant differences in protein dynamics. We report a residue-specific characterization of the thermal unfolding mechanism of cyt c using C-D bonds site-specifically incorporated at residues dispersed throughout three different structural elements within the protein. Elucidation of the detailed unfolding mechanism and the structure of the associated molten globule, both of which represent challenges to conventional techniques, are highlights of the utility of the C-D technique.
We report a residue-specific characterization of the thermal unfolding mechanism of cyt c using C-D bonds site-specifically incorporated at residues dispersed throughout three different structural elements within the protein. Elucidation of the detailed unfolding mechanism and the structure of the associated molten globule, both of which represent challenges to conventional techniques, are highlights of the utility of the C-D technique. The cyano group is sensitive to its environment, absorbs in a unique region of the protein IR spectrum, and may be appended to an amino acid. Using both steady-state and time-resolved methods, we explore the use of cyano groups as probes of protein microenvironments and dynamics in variants of cytochrome c. We find that the cyano group is a useful site-specific probe of protein microenvironments and dynamics, but that it can also perturb its environment and destabilize the folded state of the protein.
The cyano group is sensitive to its environment, absorbs in a unique region of the protein IR spectrum, and may be appended to an amino acid. Using both steady-state and time-resolved methods, we explore the use of cyano groups as probes of protein microenvironments and dynamics in variants of cytochrome c. We find that the cyano group is a useful site-specific probe of protein microenvironments and dynamics, but that it can also perturb its environment and destabilize the folded state of the protein. We explore the use of carbon-deuterium (C-D) bonds under time-resolved conditions to follow the unfolding of cytochrome c from a photostationary state that accumulates after CO is photodissociated from the protein’s heme prosthetic group. Our results clearly show that C-D bonds are well-suited to characterize protein folding and dynamics.
We explore the use of carbon-deuterium (C-D) bonds under time-resolved conditions to follow the unfolding of cytochrome c from a photostationary state that accumulates after CO is photodissociated from the protein’s heme prosthetic group. Our results clearly show that C-D bonds are well-suited to characterize protein folding and dynamics.